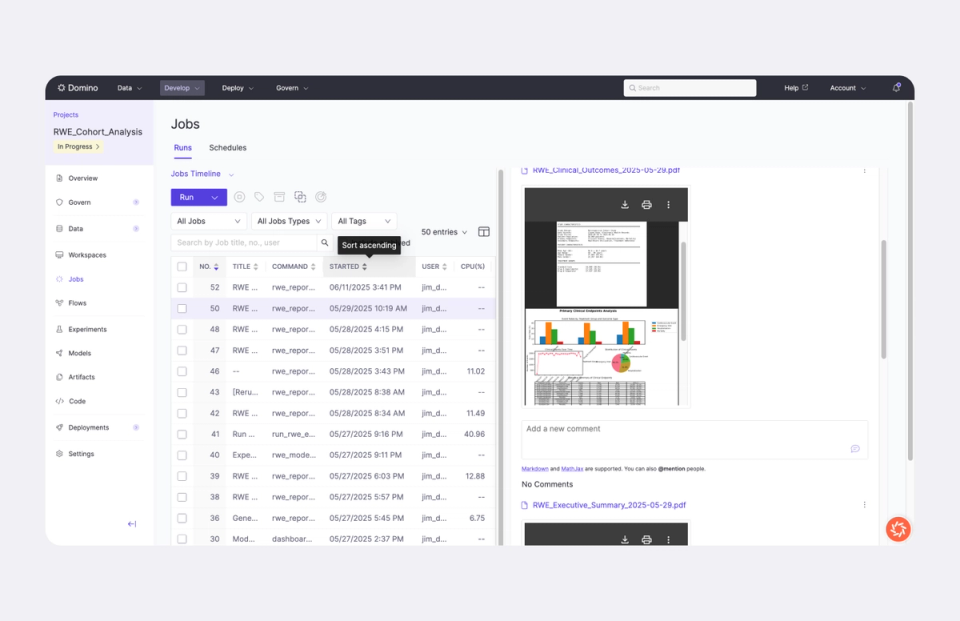
Unified environment for real‑world data
Ingest and join structured and unstructured data, including 100M+ patient records, using integrated Spark workflows. Analyze at scale without switching tools or compromising performance.
Real‑world evidence is only as strong as the platform behind it. Domino provides a foundation for high‑impact science by accelerating insights, improving consistency, and ensuring traceability from data to decision.
Analyze diverse real-world data in one environment to accelerate timelines so evidence can shape trial design, payer strategy, and go/no-go decisions.
Build in R, Python, or SAS and reuse across functions and therapeutic areas. Standardize workflows to reduce duplication, improve consistency, and drive enterprise-wide impact.
Track every step from data to results with full traceability to meet regulatory requirements and maintain scientific momentum.
Deliver interactive, self-service apps so stakeholders can explore evidence directly without waiting on slides or rework.

Ingest and join structured and unstructured data, including 100M+ patient records, using integrated Spark workflows. Analyze at scale without switching tools or compromising performance.
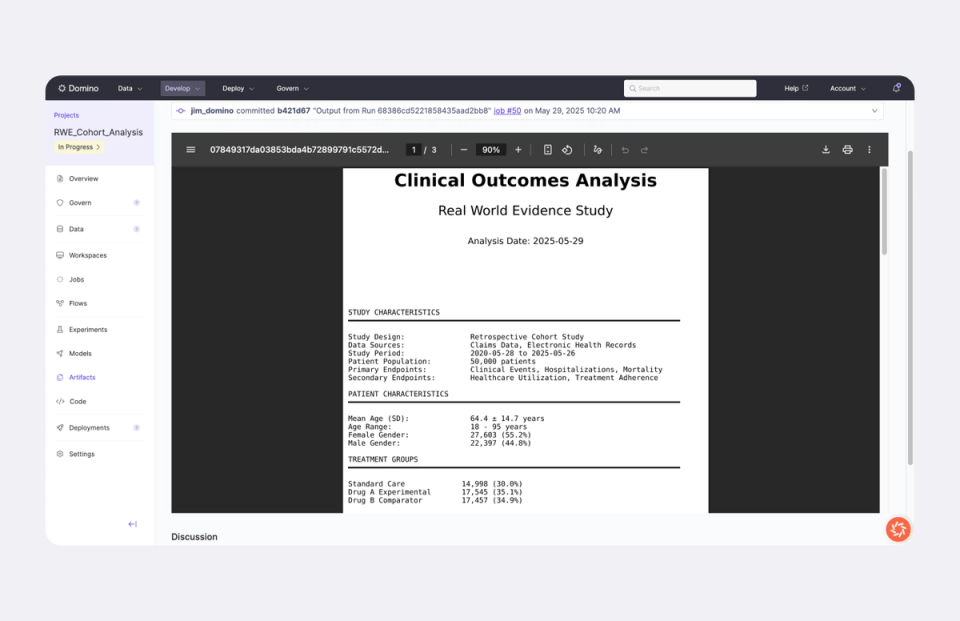
Manage multiple data sources in one secure workspace with role-based access and version control. Automatically capture lineage and export audit trails to meet 21 CFR Part 11, GxP, and HIPAA requirements.

Build modular code and workflows in R, Python, or SAS and operationalize them across studies, indications, and therapeutic areas. Reuse validated pipelines without reengineering.

Deploy predictive models as APIs to power treatment response prediction, patient risk scoring, and cost forecasting. Embed model outputs into interactive tools that support real-time decision-making across teams.

Build interactive apps to explore patient cohorts, treatment patterns, and comparative outcomes. Let stakeholders engage with results directly without needing code or reruns.
Discover how Domino helps RWE teams move faster, reuse analytics, and deliver submission-ready evidence with full compliance so insights can shape decisions, not just reports.
